Abstract
Background: CAR-modified T cells targeting CD19 have produced remarkable responses in relapsed/refractory B-cell acute lymphoblastic leukemia (B-ALL); however, relapse continues to be a substantial challenge. CD19+ relapses, which account for 33-78% of relapses, are associated with loss of CAR T-cell surveillance due to short persistence. Thus, strategies to improve functional persistence to prevent and treat CD19+ relapsed disease are crucial. Here, we report our experience administering reinfusions of murine or humanized 4-1BB CD19 CAR T cells in an effort to prolong persistence in patients with demonstrated short persistence to mitigate relapse risk, treat CD19+ relapsed disease, and produce responses after nonresponse to initial CAR infusion.
Methods: This analysis included patients aged <30 years treated with a murine CD19 CAR construct, either investigational CTL019 (NCT01626495, NCT02906371) or commercial tisagenlecleucel, or a humanized CD19 CAR construct, huCART19 (NCT02374333), who received ≥1 reinfusion of the same CAR product due to: 1) clinical signs of poor persistence within 6 months (mos) of initial infusion, including peripheral B-cell recovery (BCR) or CD19+ hematogones in the bone marrow, 2) new CD19+ minimal residual disease (MRD) or relapse, or 3) nonresponse to initial infusion. The huCART19 trial included patients who had previously received a CAR T cell product (CAR-exposed), whereas all patients reinfused with CTL019/tisagenlecleucel were CAR-naïve at initial infusion. The primary outcome was complete response (CR) at day 28 after reinfusion, defined as complete remission with establishment or maintenance of B-cell aplasia. Secondary outcomes included CRS incidence, cumulative incidence of relapse (CIR) and overall survival (OS).
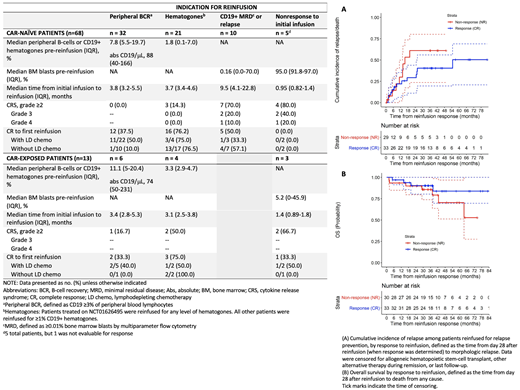
Results: Among 229 CAR-naïve and 33 CAR-exposed patients treated with CD19 CAR between 2012-2020, 81 received ≥1 reinfusion (investigational CTL019, n=44; commercial tisagenlecleucel, n=11; huCART19, n=13 CAR-naïve and n=13 CAR-exposed). In addition, 18 patients received PD-1 blockade after their first (n=11) or subsequent (n=7) reinfusions. Indications for first reinfusion were peripheral BCR (CAR-naïve, n=32; CAR-exposed, n=6), hematogones (CAR-naïve, n=21; CAR-exposed, n=4), CD19+ MRD/relapse (CAR-naïve, n=10, CAR-exposed, n=0), and nonresponse to initial infusion (CAR-naïve, n=5, CAR-exposed, n=3). CRS grade ≥2 (Penn scale) occurred in 19 patients (grade 2, n=13; grade 3, n=4; grade 4, n=2). Grade 3-4 events only occurred in patients with active disease at time of reinfusion. Twenty-two patients had an inpatient admission within 30 days of first reinfusion, of which 7 required intensive care unit admission
Among the 63 patients reinfused for relapse prevention, 33 (52%) had a CR at day 28. With a median duration of follow-up of 38 mos, 13 experienced a subsequent relapse (7 CD19+, 4 CD19-, 2 CD19-subset negative), 4 received alternative therapy or allogeneic hematopoietic stem cell transplantation (HSCT) in remission, and 16 remain in remission without further therapy at a median of 39 mos after first reinfusion. The median duration of B-cell aplasia was 8 mos (IQR 2-35) after reinfusion. Of the 30 with no response (NR), 10 had a subsequent CD19+ relapse, 15 received alternative therapy or HSCT, and 5 remain in remission without further therapy at a median of 43 mos after reinfusion. CIR and OS were not statistically significantly different between patients with CR or NR (CIR, p=0.26; OS, p=0.25) (Figure A-B). However, at 24 mos after reinfusion, CIR was 29% (95% CI, 11-44%) for CR compared to 61% (95% CI, 24-80%) for NR; OS was 90% (95% CI, 80-100%) for both groups.
Of the 10 patients reinfused for relapse, 5 (50%) had a CR; 2 subsequently experienced a CD19+ relapse, 2 received an HSCT in remission, and 1 remains in remission without further therapy at 18 mos after reinfusion. Of the 8 patients reinfused for nonresponse to initial infusion, 7 were evaluable; none had a CR, and all died at a median of 2.5 mos after reinfusion.
Conclusions: Reinfusion of CTL019/tisagenlecleucel or huCART19 is safe, may prolong B-cell aplasia in patients with short CAR persistence and reduce relapse risk, and can induce remissions in patients with CD19+ relapsed disease. Thus, reinfusion may provide an alternative to HSCT for short persistence. However, reinfusion is not effective for patients with nonresponse to initial CAR infusion.
Callahan: Novartis: Speakers Bureau. Rheingold: Optinose: Other: Spouse's current employment; Pfizer: Research Funding. June: Tmunity, DeCART, BluesphereBio, Carisma, Cellares, Celldex, Cabaletta, Poseida, Verismo, Ziopharm: Current equity holder in publicly-traded company; Novartis: Patents & Royalties; AC Immune, DeCART, BluesphereBio, Carisma, Cellares, Celldex, Cabaletta, Poseida, Verismo, Ziopharm: Consultancy. Grupp: Novartis, Roche, GSK, Humanigen, CBMG, Eureka, and Janssen/JnJ: Consultancy; Novartis, Adaptimmune, TCR2, Cellectis, Juno, Vertex, Allogene and Cabaletta: Other: Study steering committees or scientific advisory boards; Novartis, Kite, Vertex, and Servier: Research Funding; Jazz Pharmaceuticals: Consultancy, Other: Steering committee, Research Funding. Maude: Novartis Pharmaceuticals Corporation: Consultancy, Research Funding; Wugen: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal